Lesson Video: Electron Shells
Video Transcript
In this video, we will learn about
the electron shell model and how to use electron shell diagrams. We’ll describe how electrons are
arranged in the shells of atoms and ions and convert back and forth between electron
shell notation and electron shell diagrams.
We know there are many types of
atom or ion called elements. Inside the nucleus, we find protons
and neutrons. And it’s the number of protons that
determines the element. If we count up the protons, we’ll
get the atomic number for the element. We can find lots of information
about the elements on the periodic table of elements. Let’s take the element carbon as an
example. The atomic number of carbon is six,
which means atoms or ions of carbon contain six protons. So, if we have an atom or ion of
carbon, we know for sure it contains six protons, but we don’t know for sure the
number of neutrons, but we are going to look at that in this video.
What’s more important is to
remember that protons are positively charged, which make the nucleus positively
charged. And the nucleus strongly attracts
negative electrons. Electrons and protons have equal
but opposite charge, meaning that to form a neutral atom, we need exactly the same
number of electrons as we have protons. If we have more electrons or fewer
electrons than the number of protons, then we have an ion with an overall
charge. If there are more electrons than
protons, then we have a negatively charged anion. But if we have fewer electrons than
protons, then we have a positively charged cation.
Here are the steps you’d have to
follow to work out the number of electrons in an atom or ion. The first thing you’ll have to do
is determine the number of protons in the atom or ion. You might need to count them in a
diagram or use the atomic number found by looking at the element’s symbol. Then, the number of electrons is
simply equal to the number of protons, at least for an atom. For an ion, however, you would add
or remove electrons for the charge, adding electrons for an anion or taking them
away for a cation.
Let’s run this through with an
example, Mg2+. We can look up Mg on the periodic
table and see that the atomic number for magnesium is 12. So, we have 12 protons. Then, we convert the number of
protons to the number of electrons, and we’d have 12 electrons if we were dealing
with a magnesium atom. But we’re dealing with a magnesium
two plus cation, so we take two electrons away, leaving us with 10 electrons.
Now we know how to count the
electrons in an atom or ion, let’s have a look at how they’re arranged.
We have known for a long time that
electrons aren’t just orbiting at random around the nucleus. Some electrons are closer to the
nucleus than others. And the way they move and interact
is very complicated. Quantum theory can describe the
bizarre way that electrons move around the nucleus. However, neither of these models
are the best tools for understanding the basics of chemistry, so we need a model in
between.
That’s the electron shell
model. In this model, electrons are said
to occupy shells around the nucleus. Each shell is further from the
nucleus than the last. Like layers of an onion, they get
bigger and bigger and bigger. The shells further from the nucleus
can fit more electrons. And lastly, electrons are more
stable if they’re in a shell closer to the nucleus. This model is only really effective
for the first 20 elements. Beyond this, it doesn’t tell us
enough about how electrons really behave.
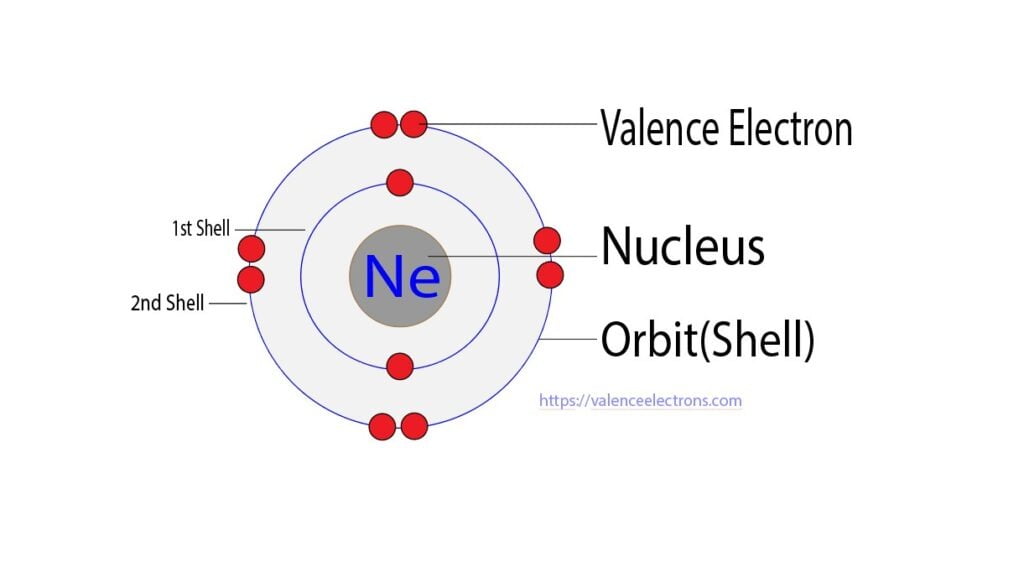
The very first electron shell is
the one that’s closest to the nucleus, and it’s quite small and can fit only two
electrons. We can draw an atom of hydrogen or
an atom of helium like this. The electron configuration is how
we write out the number of electrons in each shell. For hydrogen, it’s simply one. For helium, it’s two. There’s not enough space in the
first shell for any more electrons. So, for particles with three or
more electrons, we need to go to the second shell.
The second electron shell is
further from the nucleus, it’s bigger, and it can fit eight electrons. We can draw atoms of lithium,
beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon like this. Lithium has an atomic number of
three, so an atom of lithium has three electrons: two in the first shell and one in
the second. For atoms of the other elements
here, we’re simply adding one electron to the outer shell until we have a total of
eight. Now that we filled the second
electron shell, it’s time to move on to the third.
The third electron shell is even
bigger. In this simple model, it can
contain a maximum of eight electrons. At higher levels, you may be told
that it can fit 18. This is done when we include the
first row of the D-block of the periodic table, scandium to zinc. But this is where the model breaks
down. You don’t need to worry about this
for now. Just assume you’ll never need to
use this model beyond calcium. Atoms of sodium to argon have their
outer electrons in the third electron shell, with configurations two, eight, one to
two, eight, eight. Now, we can move on to the fourth
shell.
The fourth electron shell can
contain 32 electrons, but this simple model only really works for the first two. Using this simple model, we can
draw atoms of potassium and calcium like this. The electron configuration of a
potassium atom is two in the first shell, eight in the second, eight in the third,
and one electron in the fourth. Remember, you can look up the
element’s symbol in the middle of the diagram on the periodic table and use the
atomic number to determine the number of protons in the nucleus. Once you’ve drawn your diagram, you
can check you have the right number of electrons by adding up the number of
electrons in each shell.
Before we go any further, it’s
worth at this point having a look at what use the electron shell model is.
Here are the first 20 elements from
hydrogen to calcium. And we have the atomic numbers for
each element, which tell us the number of protons there are in an atom or ion of
that element. In chemistry, we want tools that
will quickly and reliably tell us how elements will react with one another. To start off with, we can have a
look at how these elements pair up. For this, we’re going to simplify
things a lot and just look at the number of bonds formed by individual atoms or the
charge of the ions they’ll form.
Helium, neon, and argon are
unreactive noble gases, so they have a combining power of zero. Hydrogen atoms tend to form single
bonds or singly charged ions. The metals lithium and beryllium
commonly form cations, lithium plus and beryllium two plus, while boron and carbon
will tend to form three or four covalent bonds, respectively. Beyond carbon, the combining power
goes down, with nitrogen tending to form three bonds, oxygen forming two, and
fluorine forming one. We can also get O2− and F−
anions. Between neon and argon, we get the
same pattern, where the combining power increases to a maximum of four for silicon
and decreases down to zero again. And looking beyond argon, we get K+
and Ca2+ ions.
If you look carefully, you should
be able to see a pattern that corresponds to the maximum number of electrons that
can fill the electron shells: two, eight, eight, and so on. We can express this pattern in a
simple principle. Atoms of any element will gain or
lose electrons or form covalent bonds until they have a full outer shell. There are some exceptions, but you
can still understand a great deal of very interesting chemistry using this simple
model. We can use the periodic table to
predict how a particular element will react.
Carbon atoms will tend to form four
covalent bonds, filling up their outer shell. On the other hand, atoms of
magnesium will tend to lose the two outer electrons in the third electron shell,
leaving a full second electron shell underneath. You don’t need to remember all this
detail, but what is helpful to remember is that this model is useful for predicting
basic chemical behavior. Now, let’s have a look at the
diagrams when we add or remove electrons.
This is how we might draw an atom
of sodium, with 11 protons in the nucleus and 11 electrons in the electron
cloud. Using our electron shell model, we
can describe in more detail how those electrons are arranged: two in the first
shell, eight in the second shell, and one in the third.
Sodium, the element, is in group
one of the periodic table. So, we would predict that an atom
of sodium is quite likely to lose its outer electron and form an Na+ ion. When removing electrons, we usually
remove the least stable electrons first, the ones in the outer shell. In the case of sodium, we produce
an Na+ ion with an empty third shell and a full second shell. The outer shell is this shell
furthest from the nucleus that still has a least one electron in it.
We see a similar situation but in
reverse for chlorine on the other side of the periodic table. The element of chlorine in group 17
of the periodic table, otherwise known as group seven, will commonly react to gain
one electron or form a single covalent bond to fill that empty space in the third
electron shell. And this is how you would draw the
chloride ion, with an electron configuration of two, eight, eight. And for the sodium ion, the
configuration is two, eight. It’s quite common to see these
diagrams combined, where we see an electron move from the outer shell of the sodium
atom to the outer shell of the chlorine atom. As you can see, the total number of
electrons, 28, stays the same.
Before we look at some examples,
I’m going to show you how electron shell diagrams might be used to illustrate
various forms of bonding. Let’s look at the covalent bonding
between two hydrogen atoms. You might see electron shell
diagrams illustrate the two electrons in between the hydrogen nuclei. Since they’re sharing electrons,
the two individual hydrogen atoms effectively have full outer shells.
A good example of an ionic
attraction is what occurs between lithium and fluorine atoms after they react. When the atoms come close enough
together, an electron from the outer shell of lithium hops over to the outer shell
of fluorine, forming Li+ and F− ions. The second electron shell of the
Li+ ion is empty, so it doesn’t need to be drawn.
Electron shell diagrams aren’t
typically used to illustrate metallic bonding, but let’s have a go anyway with two
beryllium atoms. When metal atoms bond and come
together, they lose their outer electrons and they become delocalized. However, it gets a little messy to
use electron shell diagrams to illustrate this. Instead, you will more commonly see
metal ions surrounded by a sea of delocalized electrons. Now, let’s get on with that
practice.
What is the maximum number of
electrons in the first electron shell?
An electron shell is a place around
the nucleus where an electron could be. The first electron shell is simply
the electron shell that is closest to the nucleus. The first electron shell is very
small, so it can only fit two electrons. There aren’t very many ways of
simply remembering this, but you can nudge your memory by looking at the periodic
table.
There are only two elements in the
first period, the first row, of the periodic table. A hydrogen atom has one electron,
and a helium atom contains two. When we go to lithium, we have to
go down a row, and we have three electrons per atom. And the third electron has to go in
the second shell because the first is full. When we move down a row in the
first few rows of the periodic table, it means we’re adding the outer electrons to a
new shell. So, that’s how we can remember that
the maximum number of electrons in the first electron shell is two.
Now, let’s do a question where
we’re looking at analyzing an electron shell diagram.
Which picture shows the arrangement
of electrons in an atom of oxygen?
What we’ve been given are five
electron shell diagrams. In the middle is a drawing of a
nucleus, much, much bigger in relation to the atom than it would be in real
life. The red circles with p are protons,
and the white circles with n are neutrons. The black circles are electron
shells, which can fit a limited number of electrons. And the blue dots are the
electrons.
What the question is asking for is
the picture that shows the arrangement of electrons in an atom of oxygen. Oxygen is an element that we can
find on the periodic table. There, we see that the atomic
number of oxygen is eight. This means that every atom or ion
of oxygen has eight protons in its nucleus. Atoms are neutral, which means we
need an equal number of electrons and protons. The first thing we can do is check
all of our diagrams depict eight protons and eight electrons.
All the nuclei look identical, and
they each have eight protons. This means we’re dealing with
nuclei of oxygen, and we can proceed to the next test. The easiest way to count out
electrons is to work out the electron configuration of each diagram. We do this by counting the
electrons in each shell, starting with the first shell.
In the first diagram, there are two
electrons in the first electron shell, there are two in the second, and there are
four in the third. This is the right number of
electrons, but we’ll come back to this in a moment. The second diagram has
configuration two, six. And the third
diagram has configuration zero, eight. Remember, the zero still matters
because it’s an inner shell. The fourth diagram has
configuration eight. And the last diagram has
configuration four, four.
Each electron shell has a fixed
maximum number of electrons that it can fit. You can fit two electrons in the
first electron shell and up to eight in the second. The other principle we’re going to
use to find the answer is that electrons occupy the most stable space. For electron shell diagrams, that
means the lowest available shell.
So, in the first diagram, we can
see that there are two electrons in the lowest available shell. That’s good. However, there are four electrons
in the third shell when there’s still space in the second. So, this is not the correct
diagram. In the second diagram, there are
again two electrons in the first electron shell. And the remaining six out of the
eight electrons fill the second shell as they should. So, this is the correct
diagram. But let’s look at the other three
just in case.
In the third diagram, there are
eight electrons in the second shell when there are two available spaces in the
first. So, this is not the correct
configuration. For the fourth diagram, all the
electrons in the first electron shell, which is six too many. And in the last diagram, there are
two too many electrons in the first electron shell. Meaning that the picture that shows
the correct arrangement of electrons in an atom of oxygen is the one with eight
protons in the nucleus, two electrons in the first shell, and six in the second.
Now, before we finish, let’s review
the key points. An electron shell is a place around
the nucleus that can fit electrons. Electrons in shells closer to the
nucleus are more stable. A maximum of two electrons can go
in the first electron shell. And a maximum of eight electrons
can go in the second shell.
In this simple electron shell
model, we can only add eight electrons to the third shell before we start adding
electrons to the fourth. In electron shell diagrams,
electrons typically occupy the lowest energy positions first, unless we’re dealing
with something like an excited state. And we can convert between electron
shell diagrams and electron configurations by counting the number of electrons in
each shell. In this case, there are two in the
first shell, eight in the second, and there’s one in the third. And if you know the element you’re
dealing with, you should be able to convert between these forms.